Physical Properties
It
is possible to theoretically predict many different molecular
properties of known and theoretical compounds. These properties include:
- atomic charges
- molecular orbitals
- vibrational (IR and Raman) spectra
- electronic (UV-VIS) spectra
- NMR chemical shifts and coupling
- Ionisation (oxidation) potentials
- polarizabilities and hyperpolarizabilities
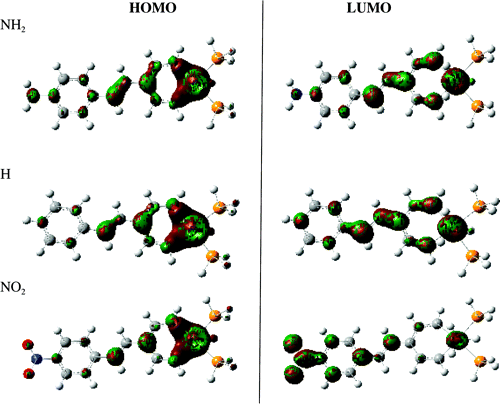
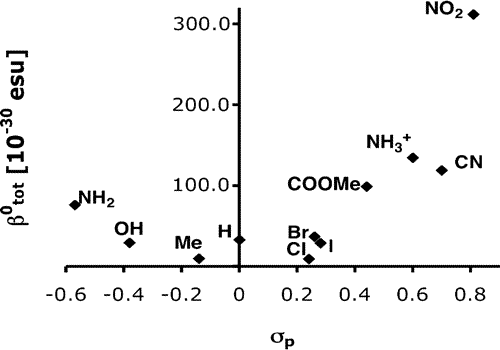
For
example, here are the HOMO and LUMO molecular orbitals of substituted
Iridium-stilbazole complexes, which was used to help rationalize the
observed trends in the hyperpolarizabilities (β
0tot) in the plot (from
Paper #12).
Kinetics and Thermochemistry
One can also calculate and predict the kinetic and thermochemical properties of molecules and molecular systems, including:
- Reaction kinetics (calculations of rate constants)
- Reaction thermochemistry
- Solvent effects
Future services:
- Reactions in Periodic Systems
- There
is a growing interest in reactions in a periodic system, such as on a
surface or in a monolayer. If any problems of this type arise, I would
be interested. In addition, Prof. Andreas Heyden
(U. of South Carolina, formerly also a post-doctoral researcher with
Prof. Truhlar, U. of Minnesota) is developing multilayer methods
("Adaptive Partitioning", see: A. Heyden, H. Lin and D. G. Truhlar, J. Phys. Chem. B 2007, 111, 2231-2241)
that allow for the transit of atoms/molecules between the different
level of a multilevel (e.g., QM/MM) calculation. These methods could
prove most useful in such an endevour and I am in close contact with
Prof. Heyden.
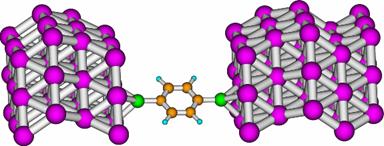
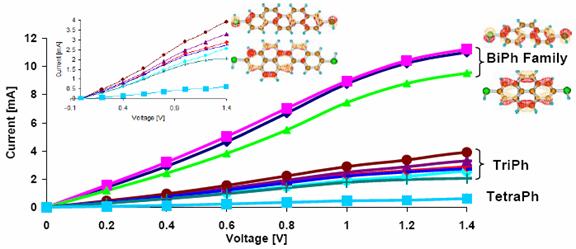
- Single-Molecule electronic transport
calculations. This was a field in which Dr. Revital Cohen had
experience. If needed, calculations along these lines may be possible.
The following is a plot from one of her papers (H. Basch, R. Cohen and M. A. Ratner, Nano Lett. 2005, 5, 1668-1675).
(last updated: November 14, 2007)